A Mole of Water Molecules Would Just About Fill a
Since we have an extremely large number the mole and these in nitesimal particles atoms a mole of atoms is a convenient quantity to work with in a laboratory. Determine the mass and record it.
How Many Molecules Are In 48 90 Grams Of Water Socratic
From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.

. Although this represents just a tiny fraction of 1 mole of water 18 g it contains more water molecules than can be clearly imagined. The wheel barrow and gallon bucket have larger volumes of which 18 mL of water will never fill them. So next we calculate how many molecules there are in a drop of water which we determined contains 0002775 moles.
A higher boiling point When ice forms the __________ are farther apart than in liquid water allowing the ice to form an organized crystal structure and float. One mole of sucrose dissolved in 2 liters of solution C. 18 mL of water will fill a table spoon but not a cup which is about 237mL.
Wipe off the outside. How many moles of H 2 O are present in 2400 g of water about the mass of a cup of water. One mole contains 602 x 10²³ atoms.
Molecules in a drop of water 6022 x 10 23 moleculesmole x 0002275 moles. Two liters of solution that contains 1 mole of sucrose E. So although we altogether have 18066 x 10 23 atoms involved they are snapped together into 6022 x 10 23 molecules made up of three snapped-together atoms.
Hydrogen bonds Many of waters emergent properties such as its cohesion its high specific heat and its high heat of vaporization result from the fact that water molecules. A mole of any substance is 6022141791023 just use 6022molecules of it. There are therefore 602 10 23 water molecules in a mole of water molecules.
A single atom can be calculated by dividing the mass of one mole by Avogadros number. Use the molar mass of H 2 O as a conversion factor from mass to moles. Just like the dozen and the gross it is a name that stands for a number.
Reweigh the paper cup and water and record its mass. A unit for expressing the number of atoms in the average atomic mass of any element. The mole is the SI unit for amount of a substance.
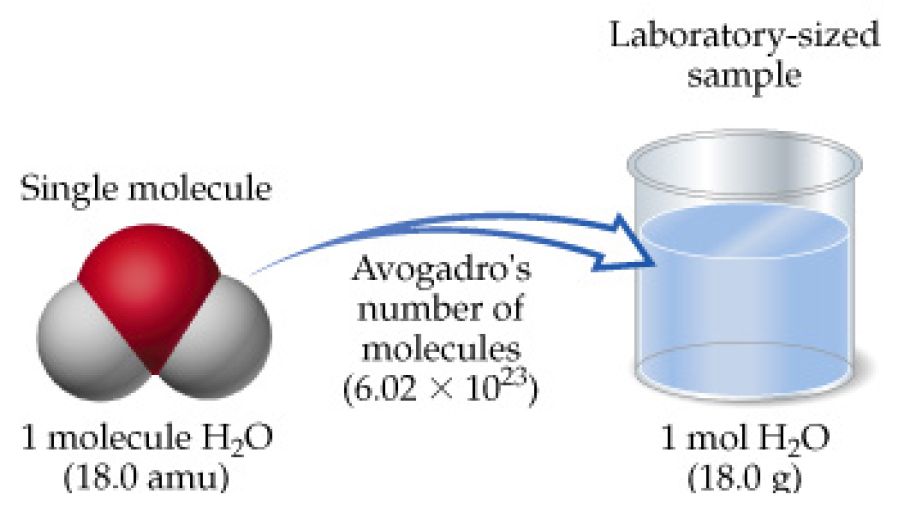
Molar Mass of water. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. The number of molecules in a single droplet of water is roughly 100 billion times greater than the number of people on earth.
Likewise in order to get 6022 x 10 23 molecules of H 2 O a mole of water we need 12044 x 10 23 which is 6022 x 10 23 x 2 atoms of hydrogen and 6022 x 10 23 atoms of oxygen. In fact a mole of sucrose table sugar weighs only 342 grams and would barely fill the volume of a tennis. Arrange the following in order of mass from the smallest mass to the largest.
10 mol Ar 30 x 1024 atoms Ne 20 g Kr. So 123 moles of any substance including wateris 123 x 6022 x 1023 molecules whatever that is. A mole of water is just 18g and thats equivalent to 18 mL.
If we were to go to a store and buy a bunch of eggs equivalent to the number of molecules in a mole we could fill the volume of the earth approximately 40 times. A mole of helium atoms has a mass of 4 grams a bit more than a peanut and a mole of lead atoms has a mass of 207 grams about the mass of a co ee mug. Absorb water store solar energy.
Because 1 mole. There also would be 602 10 23 bananas in a mole of bananas if such a huge number of bananas ever existed. Wipe off the outside.
One liter of solution that contains 602 1023 molecules of sucrose B. Thankfully molecules are much smaller than eggs. However since atoms are so tiny a mole of atoms or molecules is a perfectly manageable quantity to use in a reaction Fig.
Fill a paper cup with water. To give you some idea 1 mole of seconds represents a span of time 4 million times as long as the earth has already existed and 1 mole of marbles is enough to cover the entire earth to a depth of 50 miles. Water molecules have _____ than molecules of similar size such as ammonia and methane reflecting its capacity to absorb large amounts of heat.
Drink one mouthful of water. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs. How many water molecules are in a mouthful of water.
The molar mass of water is 10079 10079 15999 18015 gmol. Compound that has a specific number of water molecules bound to its atom. Throw away the cup.
602 x 10 23 atoms and the molar mass of an element is equal to one mole the mass of. One liter of solution that contains 2 moles of water D. Water molecules have a polarity which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as _____.
Atomic mass is the number of grams per mole of the element. Just how big is a mole. The average atomic mass in grams is the mass of one mole of atoms.
Molecules in a drop of water 167 x 10 21 water molecules.

Stoichiometry Chapter 11 12 I Things You Should Remember From The Moles Unit Identify Particles As Atoms Molecules Mc And Formula Units Fun Ppt Download

A Mole Of Water Molecules Would Just About Fill A A Large Wheelbarrow B Tablespoon C Cup D Gallon Brainly Com
No comments for "A Mole of Water Molecules Would Just About Fill a"
Post a Comment